Abstract
Background Myelofibrosis (MF) is featured by an inflammatory condition that can also drive the progression of disease. Ruxolitinib (RUX) is the-first-in-class Jak1/2 inhibitor approved for treatment for MF. Clinical benefits of RUX are presumably derived from reduction of inflammatory cytokines even if the exact mechanism remains unclear. Recent reports have identified the ratio between absolute neutrophils count (ANC) and absolute lymphocyte count (ALC), called NLR, as a simple parameter that mirrors the inflammatory status and the myeloid associated immune suppression. In various malignancies NLR has been indicated as predictor of progression free survival (PFS) and overall survival (OS). Our preliminary work in a single-center experience showed that patients with NLR>6 before RUX start had a lower chance to obtain > 50% spleen reduction in the first 12 weeks or a complete resolution of splenomegaly at 24 weeks.
Objective: We proposed to test NLR=6 as bio-marker in MF to apply into clinical practice as a possible predictor of response to RUX.
MethodsWe used two separate cohorts to validate NLR (as a continuous variable and as a cut off 6) as predictor of response to RUX bases on our preliminary data from healthy volunteers (data not shown). Cohort (#1) including 111 MF patients from MD Anderson Cancer Center treated with RUX on phase 1/2 clinical trial from 2007 to 2010; and cohort (#2) including 367 patients treated at 18 Italian centers between years 2012 - 2018.
Spleen responses to RUX treatment, PFS and OS were independently validated in cohorts #1 and 2. As cohort 1 included patients treated on clinical trial, spleen was assessed by MRI before and after 24 weeks of RUX therapy, and by physical examination at week 12. In cohort #2, spleen size was assessed by physical examination before, after 12 and 24 weeks of RUX continuous treatment in a real-life setting. NLR was calculated using data obtained from the complete blood count before RUX start and correlated with driver mutations, early spleen reduction, progression free survival (PFS), defined as time from RUX start to last follow-up or progressive disease (including progression to acute myeloid leukemia, ≥20% blasts in peripheral blood or bone marrow, AML) or death for any reason; and overall survival (OS).
Results:
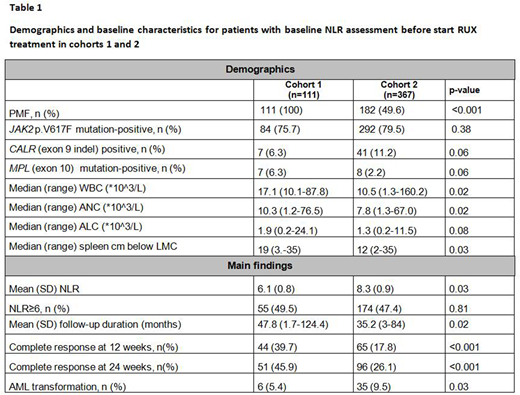
Clinical and demographics characteristics of patients in each cohort are summarized in Table 1.
In cohort #1 we found that NLR was lower in patients with lower bone marrow fibrosis (grade 0-1: 6.2±0.8 versus grade 2-3: 7.3±0.8, p=0.03). Similarly, in cohort #2, patients with grade 0-1 bone marrow fibrosis had lower NLR than those carrying grade 2-3 bone marrow fibrosis (7.7±0.7 versus 10.6±1.3, p=0.04). NLR was higher in patients carrying JAK2 (V617F) mutation (mean +/- SD, 6.4±0.6 vs 5.3±0.5, p=0.02 in cohort 1 and 9.1±0.6 vs 5.0±0.5, p=0.002 in cohort 2). While in cohort 1 NLR appeared lower in CALR (exon 9 indel) mutated patients, the difference was statistically significant in cohort 2 (5.4±0.8 vs 8.9±0.6, p=0.03). In both cohorts, there were no differences in NLR in either triple negative or MPL (exon 10) patients.
In cohort 1, the mean percentage change from baseline in palpable spleen length was −47.7% at week 12 and −53.4% at week 24. NLR=6 was able to identify at baseline early response to RUX with 66.9% sensitivity and 72.3% specificity (HR 1.68, p=0.01). Patients with NLR>6 before RUX start had a lower chance to obtain a complete resolution of splenomegaly at 24 weeks (p=0.001).
These observations were confirmed in cohort 2 where NLR > 6 was able to identify at baseline early response to RUX with 50.3% sensitivity and 67.7% specificity (HR 1.56, p=0.01). The mean percentage change from baseline in palpable spleen length was −60.3% at week 12 and −66.7% at week 24. Patients with NLR>6 before RUX start had a lower chance to obtain a complete resolution of splenomegaly at 24 weeks (p<0.002).
At the time of this analysis, 84/111 (75.6%) patients in cohort 1 and 122/367 (33.2%) in cohort 2 had died (p<0.001). Progression to AML occurred in 6/111 (5.4 %) patients of cohort 1 and in 35/367 (9.5%) patients of cohort 2 (p=0.03). With median follow-ups of 47.8 months and 35.2 months for cohorts 1 and 2, respectively, NLR as a continuous variable or NLR> 6 was not a predictor of PFS or OS.
Conclusions: NLR before RUX start could serve as a useful, simple and early predictor of spleen response in MF patients; and it positively correlates with JAK2 mutation and higher fibrosis grade.
Palandri:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vitolo:Sandoz: Speakers Bureau; Takeda: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Speakers Bureau. Cuneo:Roche: Other: advisory board, Speakers Bureau; Abbvie: Other: advisory board, Speakers Bureau; Gilead: Other: advisory board, Speakers Bureau; janssen: Other: advisory board, Speakers Bureau. Aversa:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Basilea: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria. Cavo:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Palumbo:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Di Raimondo:Takeda: Honoraria, Research Funding; Celgene: Honoraria. Verstovsek:Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Italfarmaco: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal